Abstract
Background: In the GLOW study (NCT03462719), fixed-duration Ibr+Ven demonstrated superior progression-free survival (PFS), deeper, better sustained undetectable minimal residual disease (uMRD) responses, and fewer patients (pts) requiring subsequent anti-cancer treatment versus Clb+O in older and/or comorbid pts with previously untreated chronic lymphocytic leukemia (CLL; Kater AP, et al. NEJM Evidence. 2022). Unmutated IGHV (uIGHV) and TP53 mutations are risk factors associated with worse outcomes for both chemoimmunotherapy and venetoclax plus anti-CD20 therapies for CLL. Here we investigate MRD kinetics and outcomes of Ibr+Ven in GLOW according to these risk factors.
Methods: Pts aged ≥ 65 years or 18 to 64 years with cumulative illness rating scale score > 6 or creatinine clearance < 70 mL/min were enrolled and randomized 1:1 to Ibr+Ven (3 cycles of Ibr lead-in, followed by 12 cycles of Ibr+Ven) or 6 cycles of Clb+O, with a cycle defined as 28 days. Pts with del(17p) or known TP53 mutations at screening were excluded, and central evaluation of TP53 mutational status was performed during the study. The primary end point was PFS by independent review committee. Among pts with partial response or better, MRD in peripheral blood was evaluated using next-generation sequencing via clonoSEQ on-treatment and at 3-6 month intervals post-treatment. uMRD results are reported in peripheral blood (PB) at < 10-4 unless otherwise noted. Since the primary analysis, a post hoc retrospective reclassification of the IGHV status of baseline samples was conducted to reduce the number of unknowns. We used clonoSEQ data as part of CLL clonal testing (Adaptive Biotechnologies, Seattle, WA).
Results: There were 106 pts randomized to Ibr+Ven and 105 to Clb+O. In the Ibr+Ven arm, uMRD rates increased from 46.2% after 6 cycles of the combination to 54.7% at 3 months after end of treatment (EOT+3), demonstrating that the majority of disease clearance in the PB occurred early, during the first 6 months of combination treatment. Post-treatment, 77.6% (45/58) of pts in the Ibr+Ven arm sustained their uMRD status from EOT+3 to EOT+18, compared with 12.2% (5/41) in the Clb+O arm. In the Ibr+Ven arm, 17/24 patients with intermediate MRD ≥ 10-4 to < 10-2 at EOT+3 did not worsen through EOT+18. Among pts with detectable MRD (≥ 10-4) at EOT+3, 6.5% (2/31) clinically progressed by EOT+18 in the Ibr+Ven arm versus 68.1% (32/47) in the Clb+O arm.
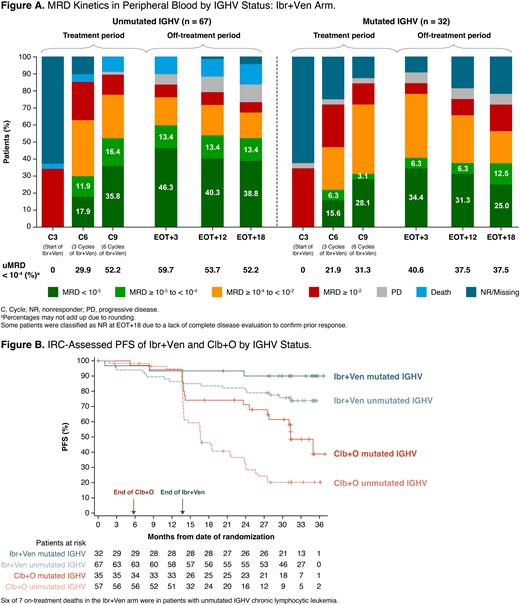
After reclassification of baseline samples, uIGHV / mutated IGHV (mIGHV) / unknown status was 63.2%/30.2%/6.6% in the Ibr+Ven arm and 54.3%/33.3%/12.4% in the Clb+O arm. In the Ibr+Ven arm, rates of uMRD after 6 cycles of combination treatment and at EOT+3, respectively, were 52.2% and 59.7% in pts with uIGHV and 31.3% and 40.6% in pts with mIGHV (Fig A). On-treatment MRD kinetics differed by IGHV status in the Ibr+Ven arm, with uMRD achieved at a higher rate and earlier in pts with uIGHV.
In the Ibr+Ven arm, uMRD was sustained post-treatment (from EOT+3 and EOT+18) in 80.0% (32/40) of pts with uIGHV and 76.9% (10/13) of pts with mIGHV. Among pts with detectable MRD (≥ 10-4) at EOT+3, 2/16 with uIGHV and 0/14 with mIGHV had clinically progressed by EOT+18 in the Ibr+Ven arm; corresponding rates were 83.3% (25/30) and 42.9% (6/14) in the Clb+O arm. Five of 7 pts with TP53-mutated CLL achieved uMRD at EOT+3 in the Ibr+Ven arm; TP53 variant allele frequencies ranged between 5.9% and 29.6% for these 5 pts and all maintained uMRD through EOT+18.
With a median study follow-up of 34.1 months, PFS rates after IGHV reclassification were well sustained post-treatment regardless of IGHV status in the Ibr+Ven arm, while uIGHV pts in the Clb+O arm relapsed more quickly (Fig B).
PFS, overall survival, and subgroup analyses with longer study follow-up will be presented.
Conclusion: All-oral, once-daily, fixed-duration Ibr+Ven achieved uMRD responses that were better sustained than Clb+O during the first 18 months post-treatment. Notably, with Ibr+Ven, uMRD rates were higher and achieved earlier in pts with uIGHV CLL versus mIGHV CLL, while uMRD was similarly sustained post-treatment irrespective of IGHV status. Clinical progressions in the first 18 months post-treatment with Ibr+Ven were uncommon even among pts with detectable MRD ≥ 10-4. MRD kinetics and sustained responses demonstrate strong efficacy of the fixed-duration Ibr+Ven combination in older pts with high-risk genomic features.
Disclosures
Niemann:AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Novo Nordisk Foundation: Research Funding; Roche: Consultancy; CSL Behring: Consultancy; Genmab: Consultancy; Takeda: Consultancy; Octapharma: Consultancy. Munir:Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria; Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees. Moreno:Janssen, Abbvie: Research Funding; Abbvie, Janssen, AstraZeneca, Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Owen:Abbvie, AstraZeneca: Consultancy, Honoraria; Janssen, Roche, Merck, Gilead, Servier: Honoraria. Follows:Roche: Consultancy, Speakers Bureau; Janpix: Consultancy; Abbvie: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Lilly: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau. Benjamini:AbbVie,Janssen,Astrazeneca: Consultancy. Janssens:Genmab: Current Employment; Abbvie: Consultancy, Other: Travel Grants, Speakers Bureau; Amgen: Consultancy, Other: travel grants, Speakers Bureau; Astra-Zeneca: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Sobi: Consultancy, Speakers Bureau; Sanofi Genzyme: Consultancy, Speakers Bureau; Roche: Consultancy; Celgene: Other: travel grants. Levin:AbbVie, Roche and Janssen: Other: Travel expenses. Robak:Abbvie: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; OctaPharma: Honoraria, Research Funding; Regeneron: Honoraria, Research Funding; GSK: Honoraria, Research Funding. Šimkovič:Janssen-Cilag, Gilead, Roche, AstraZeneca, Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants. Voloshin:Janssen, Abbvie, Sanofi: Honoraria, Other: Clinical trials, Non-financial support; Novartis, Pfizer: Other: Clinical Trials, Non-financial support. Ysebaert:Abbvie, Astra-Zeneca, Janssen, Roche, Beigene, BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Qi:Janssen: Current Employment. Qi:Janssen: Current Employment. Parisi:Janssen: Current Employment. Srinivasan:Janssen: Current Employment. Schuier:Janssen: Current Employment. Baeten:Janssen: Current Employment. Howes:Janssen Research & Development: Current Employment, Current equity holder in publicly-traded company. Bennett Caces:Janssen: Current Employment. Kater:Abbvie, Astra Zeneca, BMS, Janssen, Roche/Genentech: Research Funding; Janssen, LAVA: Patents & Royalties: Pending; Astra Zeneca, BMS, Roche/Gennetech, Janssen, Abbvie, LAVA: Membership on an entity's Board of Directors or advisory committees; Abbvie, Astra Zeneca, Janssen: Other: Speakers fee; Amsterdam UMC, University of Amsterdam: Current Employment.
OffLabel Disclosure:
The combination of ibrutinib and venetoclax is an investigational treatment for patients with previously untreated CLL. Ibrutinib and venetoclax are each individually approved for previously untreated CLL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal